Who We Are
Founded in 1967 in Singapore, Xepa has grown and evolved at the cutting edge of the industry. From our modest early years when Xepa Laboratories Pte. Ltd. produced a limited range of pharmaceutical products in a small flatted factory, we have relocated twice to larger manufacturing plants in Melaka, Malaysia. Today, Xepa is a leading manufacturer of off-patent pharmaceutical products in Malaysia.

of Leadership

in One Campus



what we do
What We Do
our core values
Leadership/
Pioneering Spirit
We will continue to strengthen the leadership and pioneering spirit established by our founders by striving to lead the industry in all aspects.
Integrity
We uphold customers’ trust built by our founders in our products and we strive to maintain the highest ethical standards. We act with integrity in all manner of dealings.
Teamwork
& Respect
We work as a team to achieve shared company objectives irrespective of our backgrounds. We respect and value everyone’s opinions and feedback.
Continuous Improvement
We stay ahead in a highly competitive market and maintain our leadership position. We continue to innovate to meet the future needs of the market and customers.
Innovation
our plant
Our Plant
- Located at Cheng Industrial Estate, Melaka, Malaysia.
- 40,500 sq metres built-up area
- 7 facilities including laboratories, liquids production plant, solids production plants, warehouse and offices.
- Spread over a 10-acre site

SPP NOVO
The construction of Oral Solid Dosage manufacturing plant (SPP Novo) was completed in the last quarter of 2018 and received regulatory approval for commercial production on 16th May 2019.
accreditation

EU GMP
European Union Good Manufacturing Practice
In year 2017, Xepa was awarded EU GMP Certificate by the national competent authority of Hungary in European Union.
The scope of the EU GMP certification encompasses the manufacturing of sterile (eye drops) and non-sterile products (solids, semi-solids and liquids), packaging (both primary and secondary) and quality control testing (microbiological and chemical/physical) of human medicinal products.

PIC/s GMP
Pharmaceutical Inspection Co-operation Scheme Good Manufacturing Practice

TGA GMP
Therapeutic Goods Administration
of Australia Good Manufacturing
Practice Compliance

EN ISO 13485:2016
Manufacturing
and Distribution of Medical Device

ISO/IEC 17025:2017
Technical Competency and Management
System for Analytical Laboratory

ISO9001:2015
Quality Management System

GDPMD
Good Distribution Practice for Medical Devices

Halal Certification
MS 2424:2019 Pharmaceuticals
MS 2636:2019 Medical Devices
MS 2634:2019 Cosmetics
Islamic Law and Malaysian Halal Standard

ISO 45001:2018
Occupational Health and Safety Management
System Certified (UKAS)
ISO 45001:2018
Occupational Health and Safety Management
System Certified (ACB)

ISO14001:2015
Environmental Management System
global presence
Global Presence
Xepa’s products are exported to countries in Asia Pacific, Africa and Middle East.
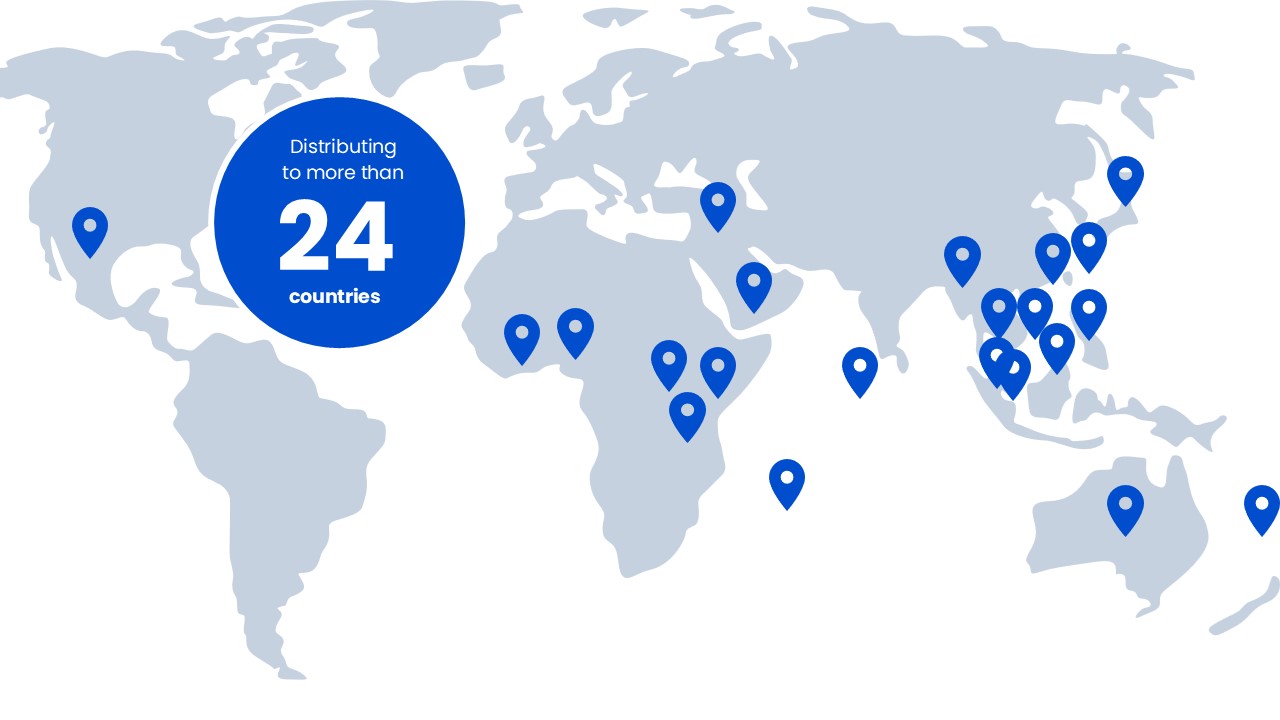
- Malaysia
- Singapore
- Vietnam
- Myanmar
- Hong Kong
- Taiwan
- Macau
- Japan
- Australia
- Philippines
- Cambodia
- Mexico
- Brunei
- Maldives
- Fiji
- Tonga
- Mauritius
- Tanzania
- Kenya
- Uganda
- Nigeria
- Iraq
- Yemen
- Ghana
helping people
to live healthier, longer and more productive lives.
Helping People
Xepa-Soul Pattinson constantly strives to make a positive impact in people’s lives by producing high quality and affordable medicines. We pursue a Corporate Social Responsibility (CSR) programme that extends our reach to our local communities with the same intent: helping people to live healthier, longer and more productive lives.
Our CSR policy comprises four core thrusts: People, Society, Environment and Governance. Guided by these pillars, we nurture and foster a spirit of community and volunteerism that promotes the virtue of placing others before self.
Empowering Lives
Training & Development
People are the cornerstone of our business. As an employer of choice, we seek to drive a strong learning culture through continuous training and development programs to help our people reach their full potential.


Training for Provisionally Registered Pharmacist (PRP)
Xepa is also recognised by the Pharmacy Board Malaysia as one of the Training Premises for Provisionally Registered Pharmacist (PRP). The PRPs are exposed to various areas relevant to pharmacy practice. This accreditation further consolidates our commitment to provide training and development for junior pharmacists in their pursuit of professional excellence.
Employee Health and Safety
We instil a culture of safety to achieve a zero-harm workplace. We improve employee well-being through programs such as Health Education, Health Check, Emergency Response Team (ERT) Bomba Training and various talks on safety measure.
.

Nurturing Communities

Improving Patient Access to High Quality Medicine
Xepa is a leading provider of pharmaceuticals to hospital, clinics and pharmacies with strong presence in Southeast Asia. We continuously strive to expand our footprint to serve and deliver the highest quality products affordably to as many people as possible.
Improving Patient Outcomes Through Education
In Xepa, we aspire to optimize patient outcomes by implementing medical education activities – SHINE (Supporting Healthcare Improvement & Nurturing Excellence). The program aims to increase awareness and management of various ailments including respiratory and dermatological conditions to achieve better diagnosis and patient outcomes.

Charitable Giving
We are committed to supporting communities by investing our resources in programs that address some of the biggest social challenges.
We help empower our local communities to thrive and to make a positive, lasting impact for the underserved.
Educational Grants
Xepa supports various educational grants to pharmacy undergraduates because we believe that education brings about positive change in the lives of individuals.

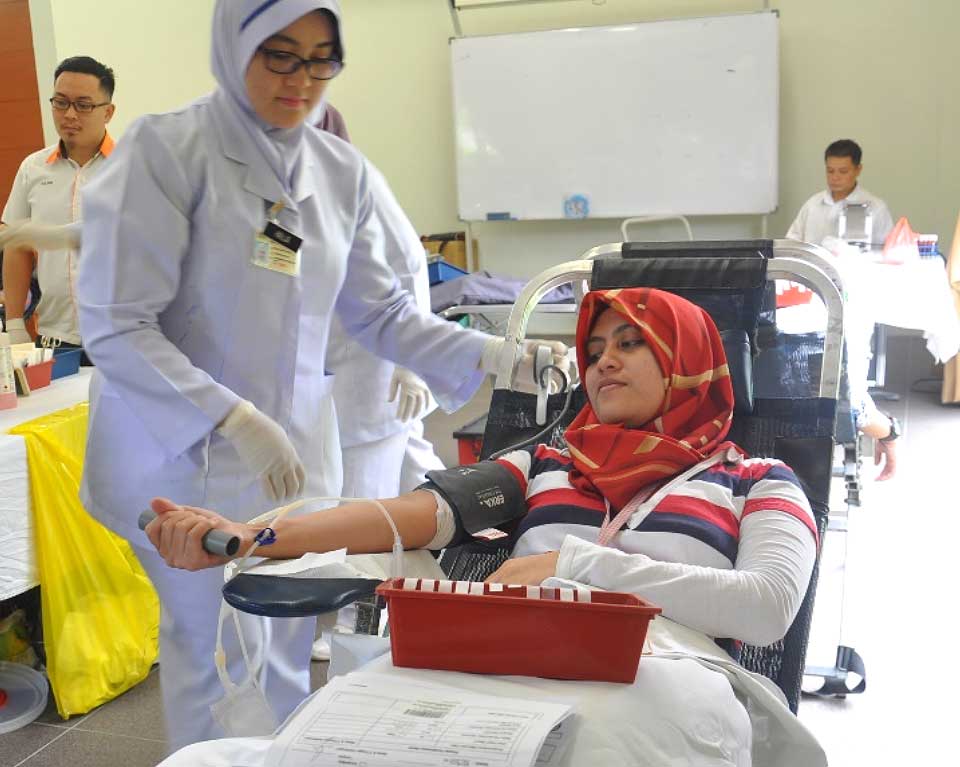
Blood Donation Drive
Xepa holds blood donation drives to meet the growing demands of blood nationally. This is part of Xepa’s Corporate Responsibility initiatives to support the National Blood Bank. It is heartening to see our staffs turning up enthusiastically to support this noble cause. The display of strength and unity was indeed applaudable.
Conserving the Environment
Waste Management & Energy Consumption
We are committed to reducing environmental footprint across the value chain, from manufacturing to distribution through recycling, proper water and waste management and increased efficiency in usage of energy while ensuring that the relevant regulations and standards are met.


Solar Renewable Energy
XEPA’s Solar Renewable Energy Project is part of the business sustainability endeavours in promoting the use of renewable energy to minimize environmental harm and reduce greenhouse gas emissions and ultimately promoting health and improving the environment.
Sustaining Growth

At Xepa, we strive to deliver long term value for our customers and shareholders. We live up to the highest standards of ethics and governance in our culture and practices.
Our management structure and governance combines responsible leadership and independent supervision.
We operate with the highest standards to ensure product safety for all.
our history
1962

1967

1973

1995

2000

2001

2002

2003

2008

2010

2013

2014

2017

2018

2020

2021

2023

2024

Today